Understanding the Molecular Pathways of Lung Cancer: Insights into Diagnosis and Targeted Therapies
Non-small cell lung cancer (NSCLC) is among the most diagnosed cancer and remains a leading cause of cancer-related mortality.
Recent advances in systemic treatments, such as immune checkpoint inhibitors (ICIs) and targeted therapies, have greatly enhanced both survival rates and health-related quality of life (HRQOL) in metastatic NSCLC patients.
Treatment options for NSCLC are primarily determined by factors like tumor grade, size, location, lymph node involvement, as well as the patient’s overall health and lung function.
Approved treatment modalities for NSCLC include surgery, chemotherapy, radiation therapy, and targeted therapies.
The treatment landscape for NSCLC is evolving rapidly, driven by the approval of new adjuvant and neoadjuvant systemic therapies.
Small cell lung cancer (SCLC) is one of the deadliest forms of cancer, with a 5-year survival rate of less than 7%.
The standard of care for extensive-stage SCLC includes systemic treatment with platinum and etoposide, now often combined with PD-L1 immune checkpoint inhibitors (ICIs).
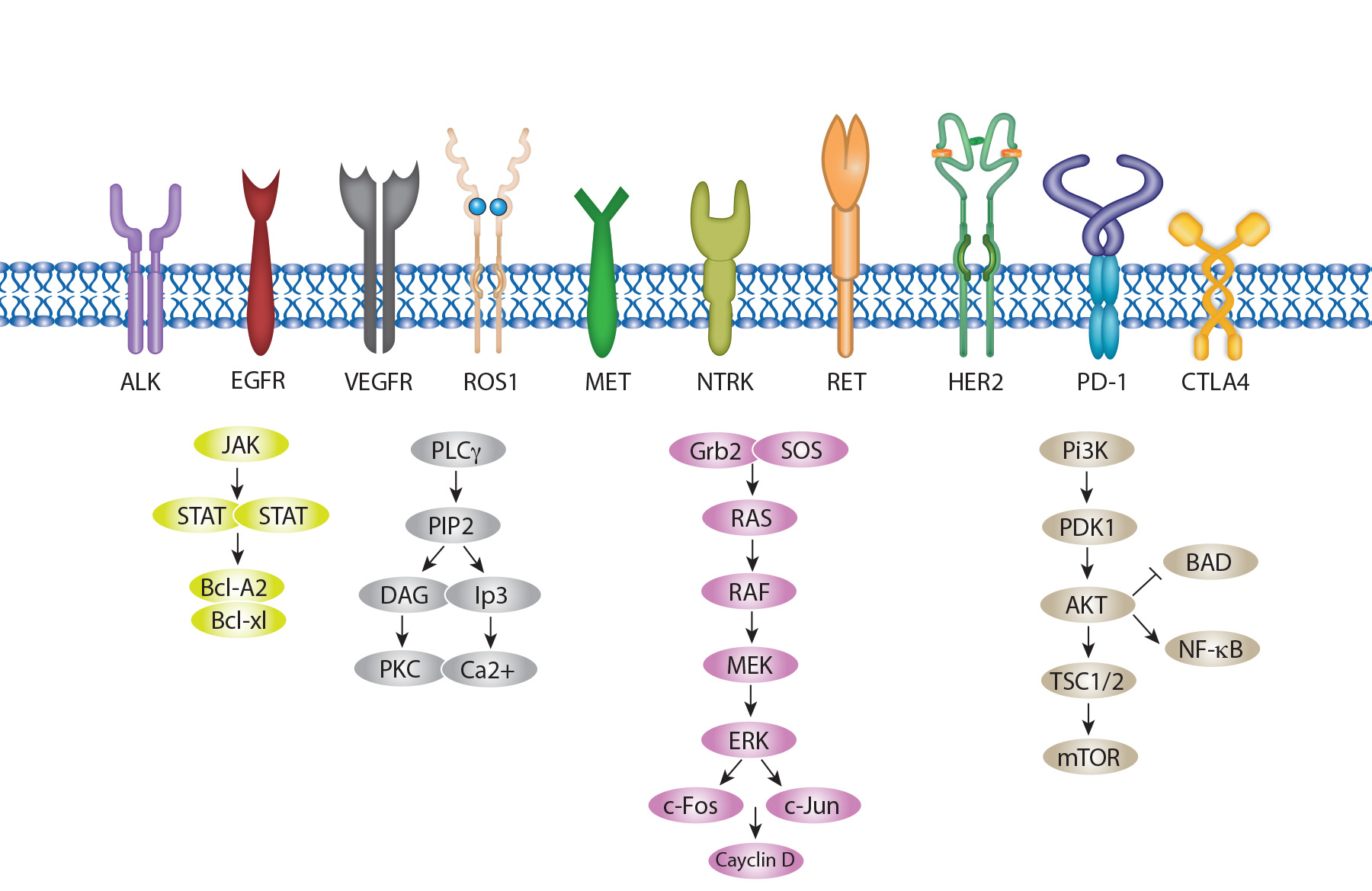
Exploring the role of Targeted Therapies in NSCLC
Lung tumors exhibit heterogeneity, with diverse oncogenic drivers and tumor microenvironments.
The advent of high-throughput molecular diagnostics has advanced precision medicine, leading to better outcomes for patients with oncogenic driver-positive NSCLC when treated with frontline targeted therapies, compared to chemotherapy and anti-PD immunotherapy.
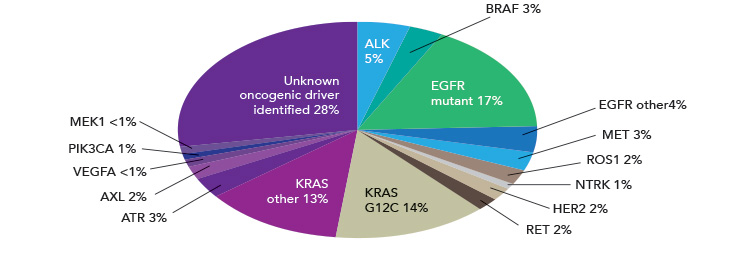
NSCLC is characterized by several common driver mutations, including EGFR amplification, ALK fusion, ROS-1 fusion, BRAF V600E mutation, and KRAS mutation.
Classic EGFR mutations (exon 19 deletions and L858R) are found in 80%–90% of lung cancer driver mutations, predominantly affecting non-smoking females of Asian descent.
On June 11, 2025, the Food and Drug Administration approved taletrectinib (Ibtrozi, Nuvation Bio Inc.), a kinase inhibitor, for adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC).
On July 2, 2025, the Food and Drug Administration granted accelerated approval to sunvozertinib (Zegfrovy, Dizal (Jiangsu) Pharmaceutical Co., Ltd.) for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
On August 8, 2025, the Food and Drug Administration granted accelerated approval to zongertinib (Hernexeos, Boehringer Ingelheim Pharmaceuticals, Inc.), a kinase inhibitor, for adults with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors have HER2 (ERBB2) tyrosine kinase domain (TKD) activating mutations
Several inhibitors targeting EGFR, KRAS, and ALK mutations are currently being investigated in clinical trials.
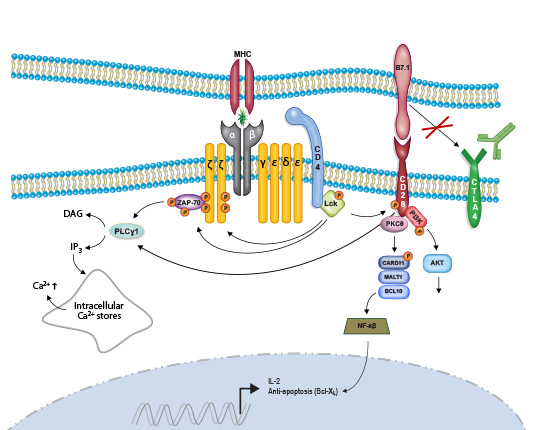
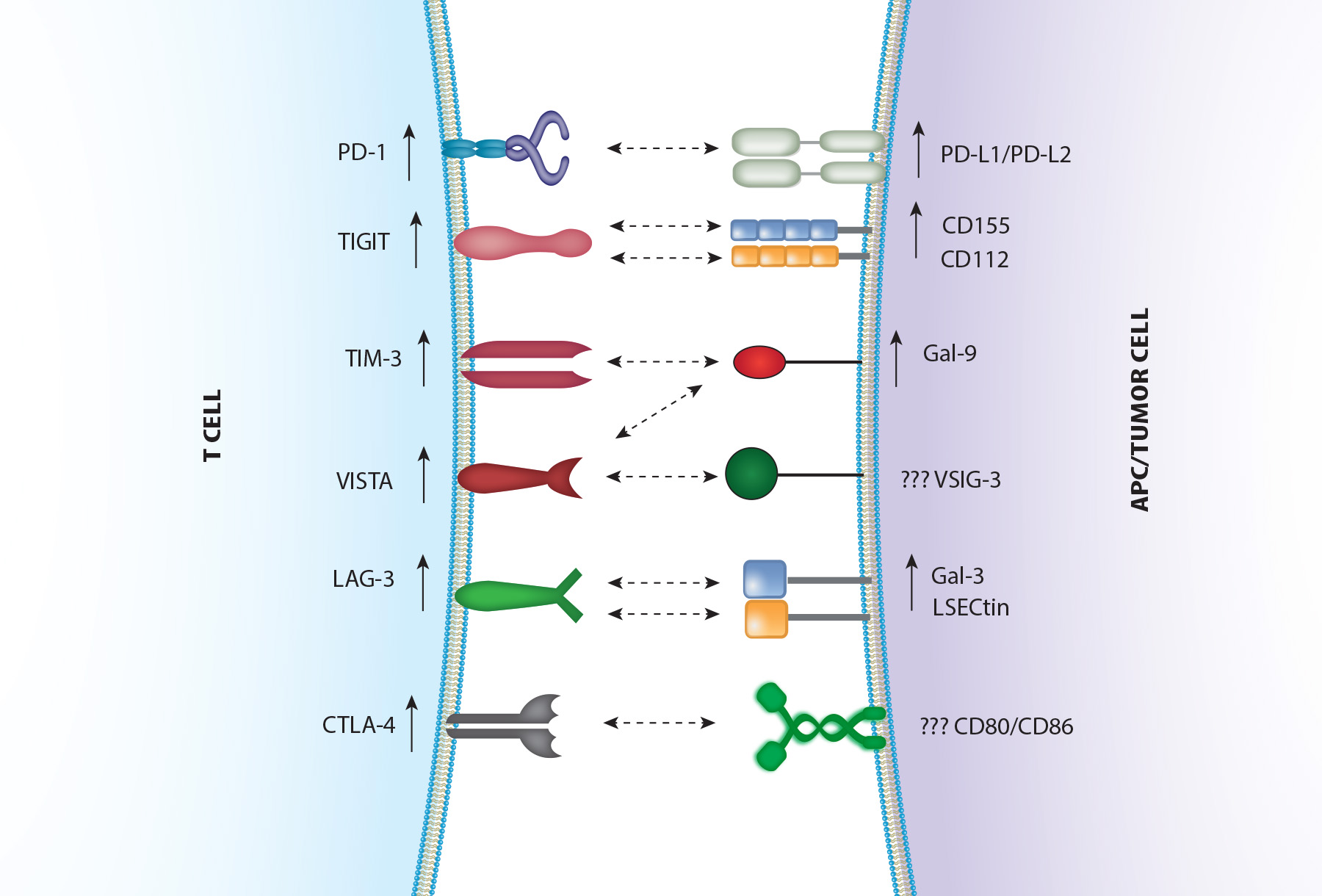
Harnessing the Power of Immune Checkpoint Inhibitors in NSCLC
Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has significantly transformed treatment outcomes, especially for advanced non-small cell lung cancer (NSCLC).
ICIs, including anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies, are now the standard of care for first-line treatment of non-oncogene-addicted metastatic NSCLC, either as monotherapy or in combination with platinum-based chemotherapy, depending on PD-L1 status.
For resectable NSCLC, multiple ICIs are under investigation in adjuvant, neoadjuvant, and perioperative settings.
On October 3, 2024, the FDA approved perioperative nivolumab (CheckMate-77T) for resectable NSCLC, following the approval of perioperative durvalumab on August 15.
On October 16, 2023, the FDA approved pembrolizumab (Keytruda, Merck) in combination with platinum-containing chemotherapy as neoadjuvant treatment and as a single-agent adjuvant therapy after surgery for resectable NSCLC (tumors ≥4 cm or node-positive), based on the KEYNOTE-671 trial.
Prior FDA approvals for NSCLC include adjuvant pembrolizumab (KEYNOTE-091), neoadjuvant nivolumab (CHECKMATE-816), and adjuvant atezolizumab (IMpower010).
On November 11, 2022, the FDA approved the combination of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) based on efficacy results from the POSEIDON trial.
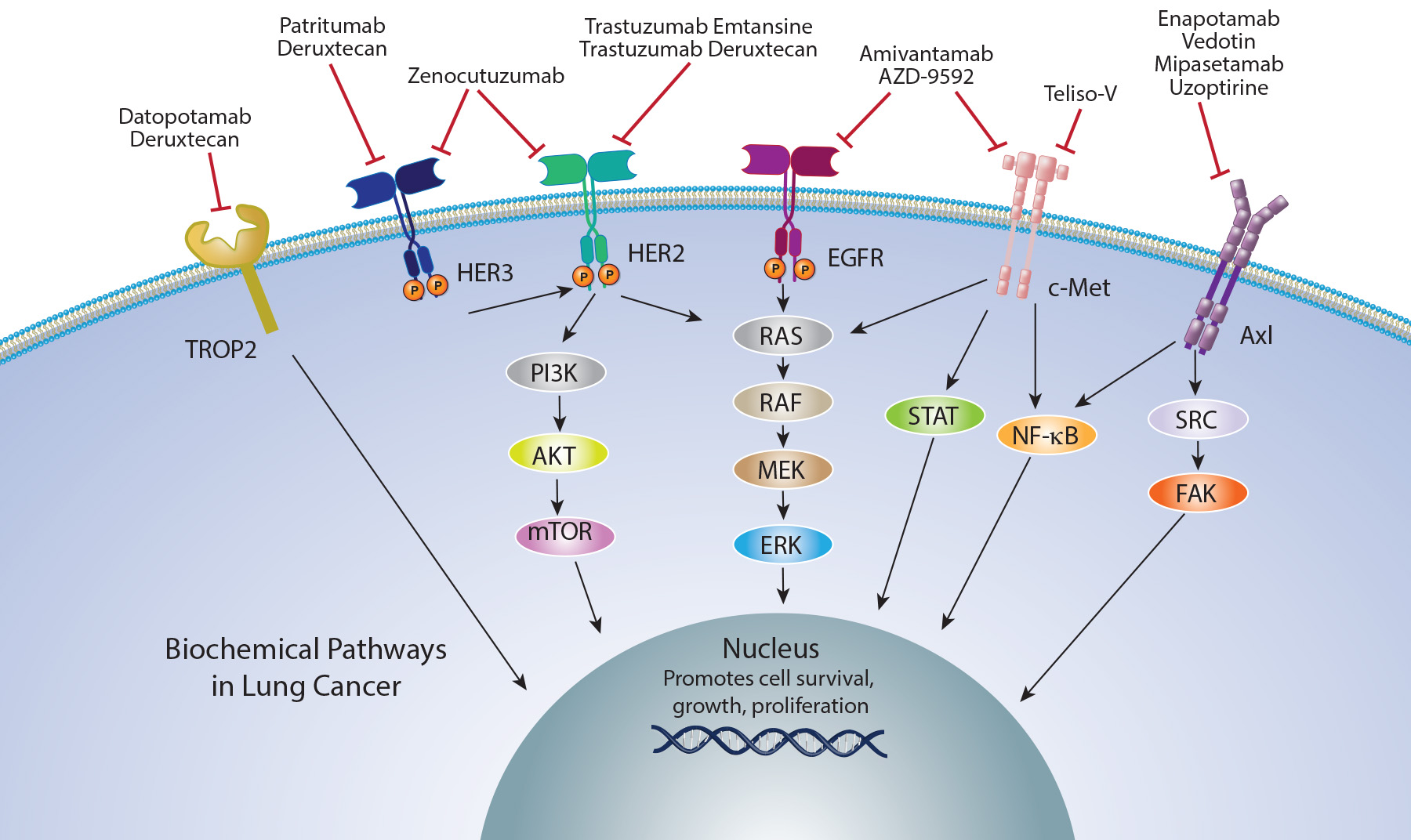
Antibody drug conjugates and Bispecific antibodies in NSCLC
Antibody Drug Conjugates (ADCs) are a novel class of therapeutics that combine an antibody targeting a tumor-specific epitope with a cytotoxic payload.
These ADCs have demonstrated significant antitumor activity across various malignancies, including lung cancer.
On the other hand, bispecific antibodies are engineered proteins with one core unit and two binding sites. Each binding site recognizes a distinct epitope, allowing bispecific antibodies to engage with two separate targets simultaneously.
The development strategies for bispecific antibodies fall into two main categories: the antigen x antigen type and the antigen x cell-engager type.
On December 4, 2024, the Food and Drug Administration granted accelerated approval to zenocutuzumab-zbco (Bizengri, Merus N.V.) for adults with advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) harboring a neuregulin 1 (NRG1) gene fusion with disease progression on or after prior systemic therapy
On May 14, 2025, the Food and Drug Administration granted accelerated approval to telisotuzumab vedotin-tllv (Emrelis, AbbVie Inc.), a c-Met-directed antibody and microtubule inhibitor conjugate, for adults with locally advanced or metastatic, non-squamous non-small cell lung cancer (NSCLC) with high c-Met protein overexpression [≥50% of tumor cells with strong (3+) staining], as determined by an FDA-approved test, who have received a prior systemic therapy.
On June 23, 2025, the Food and Drug Administration granted accelerated approval to datopotamab deruxtecan-dlnk (Datroway, Daiichi Sankyo, Inc.) for adults with locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) who have received prior EGFR-directed therapy and platinum-based chemotherapy.
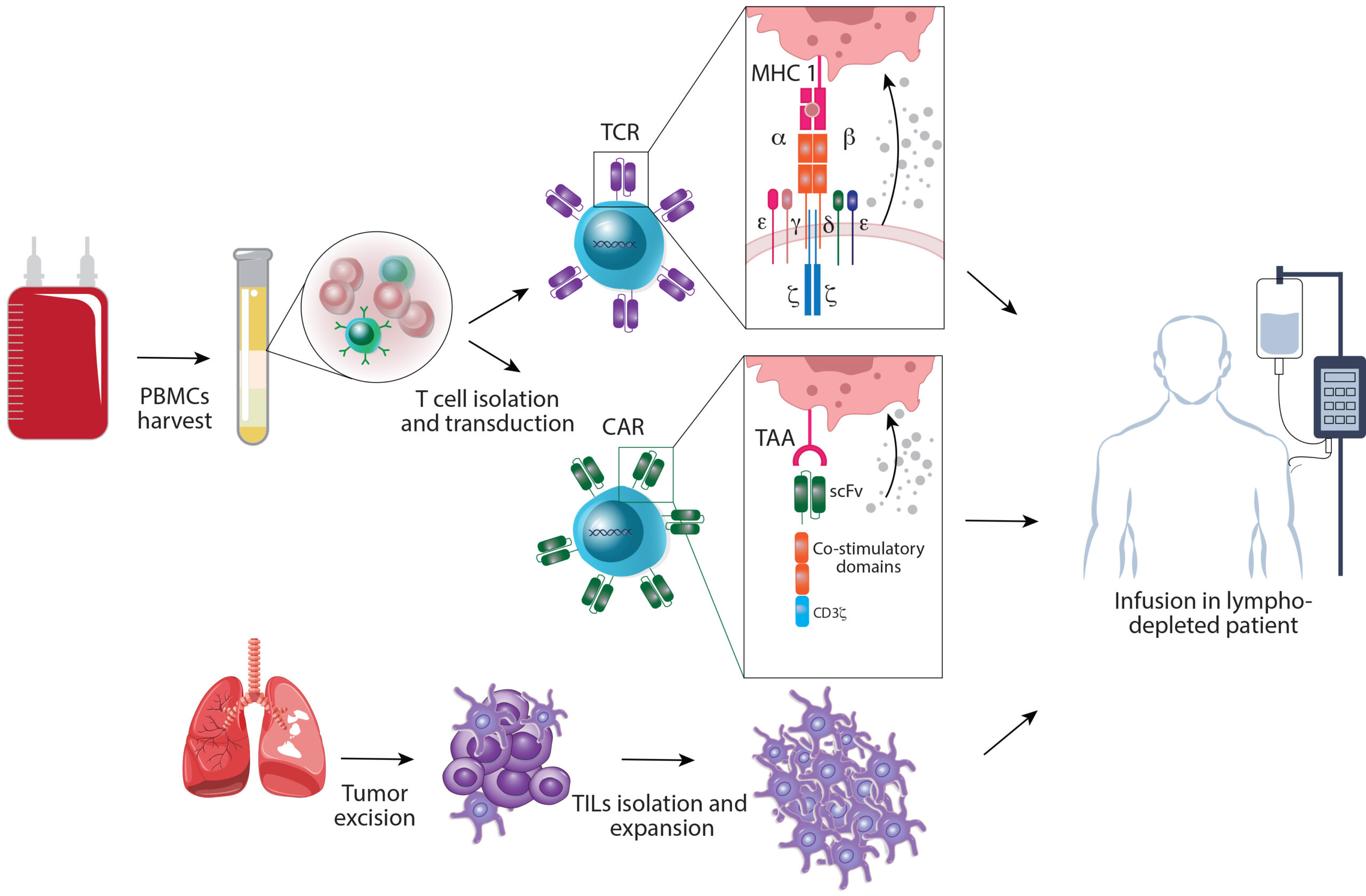
Cellular Therapies: Promising Therapeutic Agents in NSCLC
Adoptive cell therapy (ACT) approaches, including chimeric antigen receptor (CAR) T-cell therapy and tumor-infiltrating lymphocytes (TILs), are personalized immunotherapy options.
CAR T-cell therapy involves genetically modifying a patient’s T cells to express receptors that can quickly identify and attack tumor cells, while TILs focus on extracting immune cells from the tumor, expanding them in the lab, and reinfusing them into the patient.
ACT has shown promise in cancer treatment, particularly in hematologic malignancies, though its application in metastatic NSCLC (mNSCLC) is still under investigation.
Both CAR T-cell therapy and TIL therapy are in the early stages of development for lung cancer but hold potential as effective treatment options for patients with poor prognoses.
Revolutionizing NSCLC Treatment: The Crucial Role of Vaccines and Oncolytic Viruses
Cancer vaccines offer a promising approach to stimulate targeted and long-lasting immune responses against tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs).
There are four primary types of cancer vaccine platforms: cell-based, protein/peptide-based, viral vectors, and gene-based vaccines (DNA or RNA).
Cancer vaccines have the potential to induce new T cell responses against tumor antigens and enhance existing immune responses, though therapeutic vaccines have shown limited clinical benefit in NSCLC so far.
Oncolytic viruses are designed to selectively infect and replicate in tumors, leading to immunogenic cell death and attracting immune mediators to the tumor microenvironment, thereby overcoming intratumoral immunosuppression and inducing systemic antitumor immunity.
Oncolytic virotherapy operates through similar mechanisms to selectively destroy tumor cells and boost systemic antitumor immunity but, like cancer vaccines, remains in early-phase clinical trials.
Navigating Lung Challenges: Unmasking SCLC and Mesothelioma Treatment
Small-cell lung cancer (SCLC) has long been considered a recalcitrant cancer with poor prognosis, and only modest therapeutic advancements have been made over the past decades.
Immune checkpoint inhibitors have shown an ability to improve overall survival in a subset of patients with extensive-stage SCLC (ES-SCLC).
Novel cell surface targets, such as DLL3, SEZ6, TROP2, and B7-H3, along with MHC-independent immunotherapy strategies, are driving promising new clinical trials in SCLC treatment.
Significant progress in SCLC treatment includes results from the phase III ADRIATIC trial in limited-stage SCLC and the accelerated FDA approval of tarlatamab for ES-SCLC.
Malignant pleural mesothelioma, an aggressive cancer that is often unresectable, is currently treated with chemotherapy or immunotherapy as the standard of care for advanced cases.
On May 16, 2024, the Food and Drug Administration granted accelerated approval to tarlatamab-dlle (Imdelltra, Amgen, Inc.) for extensive stage small cell lung cancer (ES-SCLC) with disease progression on or after platinum-based chemotherapy.
On December 4, 2024, the Food and Drug Administration approved durvalumab (Imfinzi, AstraZeneca) for adults with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.